
COVID-19 Vaccines on the Way
We have all slipped into our routine of social distancing, masks and frequent hand washing. Most of us have limited our travel and eating out. I am sure that we all wonder how long all of this will be our “new normal”.
It helps to keep abreast of developments that promise resolution of the pandemic. Scientists, governments, and companies all over the world have moved quickly and effectively to understand the virus, develop rapid, inexpensive testing, and develop safe and effective vaccines.
Vaccine Development
There are currently over 200 vaccines under development to find a cure for the risk of COVID-19 infection.
Current Vaccines Under Development
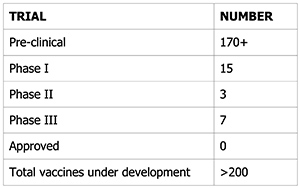
There are currently seven Phase III trials underway with thousands of people enrolled. Trials are used to confirm a vaccine’s effectiveness and safety. The companies with vaccine candidates in Phase III trials are Pfizer, Novavax, AstraZeneca, Sanofi/GSK, Moderna, Merck/IAVI, and Janssen.
Just today, Johnson & Johnson shared in a preprint the results of its Phase I/II (a) trial of its COVID-19 vaccine candidate. This trial demonstrated that 99% of all adult participants developed neutralizing antibody titers that were “in the same range” as people who recovered from COVID-19 infections. These results and a favorable safety profile have encouraged them to move ahead with their own Phase III trial.
This week the White House and FDA announced that a vaccine would likely be approved and widely available by the first of the year. A distribution infrastructure is under development for the entire population once a vaccine is shown to be safe and effective.
COVID Testing
The FDA has approved Abbott’s $5 rapid antigen called BinaxNOW COVID-19 Ag card. The test is available to health care providers for their patients within the first seven days of symptoms. The test costs about $5, returns results in 15 minutes, and the processing is self-contained in the credit card-sized package. Clinical trials have shown a 97% sensitivity/98% specificity. While it is not sold directly to consumers, the test is available from your doctor by prescription. Abbott is shipping over 10 million tests in September and is scaling up to ship 50 million tests by mid-October.
The FDA also approved the Yale SalivaDirect COVID Test. Designed for widespread public screening; this test will cost consumers about $10 per test. It is noninvasive and requires no special device, only a sterile container. The NBA, whose players used this test and compared it to the nasal swab test, confirmed this test. The test results were almost identical. The test is simple and can be performed by most labs. Consumers should be able to get results back within a few hours.
Lasting Immunity
Scientists who are studying the human immune response to the virus are seeing signs of strong, lasting immunity to the virus, even in people who developed only mild infections. These studies indicate that human immune cells are storing information about COVID so that they can fight it off again. The human response to COVID also seems much more robust and diverse, with antibodies, B cells, and T cells that all recognize the virus.
I will try to keep you informed as the science changes and update the developments in COVID management and research. – Remember vaccines are on the way.
As always, we are here for you. Please email or call if you want to set up a Zoom videoconference meeting or talk by phone.
Ralph Broadwater, M.D., CFP®, AIF®

© 2020 The Arkansas Financial Group, Inc., All rights reserved.
Please remember that past performance may not be indicative of future results. Different types of investments involve varying degrees of risk, and there can be no assurance that the future performance of any specific investment, investment strategy, or product (including the investments and/or investment strategies recommended or undertaken by The Arkansas Financial Group, Inc. (“AFG”), or any non-investment related content, made reference to directly or indirectly in this newsletter will be profitable, equal any corresponding indicated historical performance level(s), be suitable for your portfolio or individual situation, or prove successful. Due to various factors, including changing market conditions and/or applicable laws, the content may no longer be reflective of current opinions or positions. Moreover, you should not assume that any discussion or information contained in this newsletter serves as the receipt of, or as a substitute for, personalized investment advice from AFG. To the extent that a reader has any questions regarding the applicability of any specific issue discussed above to his/her individual situation, he/she is encouraged to consult with the professional advisor of his/her choosing. AFG is neither a law firm, nor a certified public accounting firm, and no portion of the newsletter content should be construed as legal or accounting advice. A copy of AFG’s current written disclosure Brochure discussing our advisory services and fees is available upon request.
Please Note: If you are a AFG client, please remember to contact AFG, in writing, if there are any changes in your personal/financial situation or investment objectives for the purpose of reviewing/evaluating/revising our previous recommendations and/or services, or if you would like to impose, add, or to modify any reasonable restrictions to our investment advisory services. AFG shall continue to rely on the accuracy of information that you have provided.
 Form CRS/ADV & Disclosures.
Form CRS/ADV & Disclosures. 